Topic: Dicyemids and chromidinids: enigmatic endoparasites
Dicyemids and chromidinids are tiny, worm-like or 'vermiform' creatures that typically live inside the kidneys ('renal organs') of cephalopod molluscs such as octopus, squid and cuttlefish.
Dicyemids and chromidinids are tiny, worm-like or ‘vermiform’ creatures that typically live inside the kidneys (‘renal organs’) of cephalopod molluscs such as octopus, squid and cuttlefish. They share numerous features, from an unusual two-phase life cycle to specialised head and body shape, all of which are related to extreme adaptation to a parasitic lifestyle. Dicyemids are dominantly parasites of benthic (sea bottom dwelling) octopuses and cuttlefish, whereas Chromidina most frequently infects oceanic or pelagic (water-column swimming) squid, cuttlefish and octopuses. Specialised infection of either benthic or pelagic hosts removes competition between the two parasite groups, and in a similar way, if multiple species are found inside a single host, they tend to show parallel anatomical adaptations of the anterior ‘head’ region, allowing each to exploit different resources or ‘niches’ within the renal appendages. In spite of their apparent similarities, the evidence points towards dicyemids and chromidinids having evolved from separate branches of the tree of life: phylum Dicyemida, Kingdom Animalia for dicyemids vs. phylum Ciliophora, Kingdom Chromalveolata (Protista) for Chromidina. This indicates convergence between the two groups, driven by strong selective pressures for successful parasitism of cephalopod renal, and occasionally pancreatic or branchial heart organs.
The dicyemid vs. chromidinid life cycle
 The dicyemid life cycle consists of asexual and sexual phases. Asexual vermiform adults (called ‘nematogens’) grow to around 0.3 – 4mm in length and live with their heads attached to the host’s heavily convoluted renal appendage epithelium. Their bodies consist of a single, elongated inner axial cell surrounded by a layer of 8-40 ciliated outer cells (the ‘somatoderm’). The axial cell is polyploid and contains diploid agametes or ‘axoblasts’ that develop into independent ‘vermiform embryos’, allowing a single nematogen to multiply into a large population asexually. When population density within the host exceeds a certain level, some vermiform embryos develop into ‘rhombogens’, bearing gonads (called infusorigens) and gametes. Fertilised eggs develop into ‘infusoriform embryos’ that are free-living and escape from the host’s body to seek a new host. Infusoriforms are made of 37-39 cells, including external ciliated cells, central germ cells and a pair of large apical cells containing ‘refringent bodies’ whose high density keeps the swimming larvae close to the sea bottom, where they can find their typical hosts of benthic octopuses and cuttlefish.
The dicyemid life cycle consists of asexual and sexual phases. Asexual vermiform adults (called ‘nematogens’) grow to around 0.3 – 4mm in length and live with their heads attached to the host’s heavily convoluted renal appendage epithelium. Their bodies consist of a single, elongated inner axial cell surrounded by a layer of 8-40 ciliated outer cells (the ‘somatoderm’). The axial cell is polyploid and contains diploid agametes or ‘axoblasts’ that develop into independent ‘vermiform embryos’, allowing a single nematogen to multiply into a large population asexually. When population density within the host exceeds a certain level, some vermiform embryos develop into ‘rhombogens’, bearing gonads (called infusorigens) and gametes. Fertilised eggs develop into ‘infusoriform embryos’ that are free-living and escape from the host’s body to seek a new host. Infusoriforms are made of 37-39 cells, including external ciliated cells, central germ cells and a pair of large apical cells containing ‘refringent bodies’ whose high density keeps the swimming larvae close to the sea bottom, where they can find their typical hosts of benthic octopuses and cuttlefish.
 Dicyemid-like chromidinids include ciliate protozoans of the genus Chromidina, whose life cycle shares parallels with dicyemids. ‘Trophonts’ resemble vermiform dicyemid nematogens in both shape and size, and live with their anterior ‘head’ attached to the renal epithelium. As in nematogens, trophonts feed (via a cell mouth or ‘cytostome’ unique to ciliophorans), grow and reproduce asexually. Trophonts repeatedly detach large elongate buds called ‘apotomites’ via transverse fission at the posterior, each apotomite growing to form a new trophont. Chromidina has a single mega-nucleus, distributed as a network throughout the cell, somewhat reminiscent of the polyploid nucleus that runs throughout the diceymid axial cell. When host age or trophont density has exceeded a certain threshold, trophonts switch to a rhombogen-like mode, developing chains of small buds or ‘tomites’ by multiple fission at the posterior of the cell. With close parallels to infusoriform larvae, tomites break off and are released as free-swimming cells, able to conjugate (exchange genetic material) and seek out their primary hosts, crustaceans. Tomites form resting cysts (phoronts) on the anterior appendages and setae of crustaceans such as Euphausia, Nyctiphanes and Thysanoessa, and only when they are eaten by their final hosts, that is the pelagic cephalopods, do they migrate to the renal appendages and develop as feeding vermiform trophonts.
Dicyemid-like chromidinids include ciliate protozoans of the genus Chromidina, whose life cycle shares parallels with dicyemids. ‘Trophonts’ resemble vermiform dicyemid nematogens in both shape and size, and live with their anterior ‘head’ attached to the renal epithelium. As in nematogens, trophonts feed (via a cell mouth or ‘cytostome’ unique to ciliophorans), grow and reproduce asexually. Trophonts repeatedly detach large elongate buds called ‘apotomites’ via transverse fission at the posterior, each apotomite growing to form a new trophont. Chromidina has a single mega-nucleus, distributed as a network throughout the cell, somewhat reminiscent of the polyploid nucleus that runs throughout the diceymid axial cell. When host age or trophont density has exceeded a certain threshold, trophonts switch to a rhombogen-like mode, developing chains of small buds or ‘tomites’ by multiple fission at the posterior of the cell. With close parallels to infusoriform larvae, tomites break off and are released as free-swimming cells, able to conjugate (exchange genetic material) and seek out their primary hosts, crustaceans. Tomites form resting cysts (phoronts) on the anterior appendages and setae of crustaceans such as Euphausia, Nyctiphanes and Thysanoessa, and only when they are eaten by their final hosts, that is the pelagic cephalopods, do they migrate to the renal appendages and develop as feeding vermiform trophonts.
Given their mode of life, dicyemids and chromidinids are often called ‘endoparasites’, implying that they are damaging to their hosts. However, it is more accurate to call them ‘endosymbionts’ as they both seem to provide benefits to host cephalopods in addition to feeding off them. H. Furuya et al. (J. Morphology, 2004, vol. 262, 629-643) suggest that beating of dicyemid and chromidinid cilia may assist in flow of fluid through the renal organ, helping with effective urine removal, and also that they make the environment more acidic, promoting toxic ammonia excretion. The parallel benefits conferred by dicyemids and Chromidina on their host highlights again their convergent form and function.
Head shape in dicyemids and chromidinids
The anterior portion or head of dicyemids is called a ‘calotte’, and its shape varies from a typical conical (type I) calotte to cap-shaped (type II), discoidal (type III) or very rarely, highly irregular (type IV) forms. Distinct calotte shapes are associated with attachment to different regions of the renal appendages, and different types appear together when there are multiple species infecting the same host, generating intense inter-specific competition for nutrients.
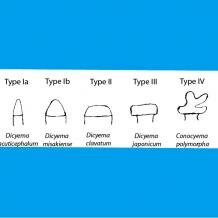 Conical, pointed calottes are most common, formed by differential growth of apical or ‘polar’ cells. Species with conical calottes attach within the deep infoldings and grooves of the renal epithelium (e.g. the recently discovered Dicyemennea sepiellae, a single species infecting Sepiella japonica). When only two species are present, one will have a conical calotte and the other typically develops a discoidal calotte that attaches to the smooth outer epithelium of the renal appendage, preventing direct competition between the species. For example, Dicyemennea abreida (conical) and Dicyemodeca deca (discoidal) commonly infect Enteroctopus dofleini as a complementary pair. Species with cap-shaped calottes, intermediate between the conical and discoidal forms, appear when more than two species are present per host. Cap-shaped calottes attach to the outer surface, exploiting a distinct niche from conical or discoidal species so that multiple forms can co-exist. An example is Dicyema colorum (cap-shaped) which infects Octopus fangsiao in various combinations with D. acuticephalum (conical), D. erythrum (conical), D. misakiense (conical) and D. japonicum (discoidal). Certain rare species, such as Dicyemennea adminicula and Microcyema vespa have highly irregular bodies and calottes. They are proposed to attach to the smooth surfaces of renal appendages, reducing competition between other species present. For example, Conocyema polymorpha (irregular calotte) has been found infecting Octopus vulgaris in the presence of three slightly different conical-calotte species, Dicyema typus, D. paradoxum and Dicyemennea lameere.
Conical, pointed calottes are most common, formed by differential growth of apical or ‘polar’ cells. Species with conical calottes attach within the deep infoldings and grooves of the renal epithelium (e.g. the recently discovered Dicyemennea sepiellae, a single species infecting Sepiella japonica). When only two species are present, one will have a conical calotte and the other typically develops a discoidal calotte that attaches to the smooth outer epithelium of the renal appendage, preventing direct competition between the species. For example, Dicyemennea abreida (conical) and Dicyemodeca deca (discoidal) commonly infect Enteroctopus dofleini as a complementary pair. Species with cap-shaped calottes, intermediate between the conical and discoidal forms, appear when more than two species are present per host. Cap-shaped calottes attach to the outer surface, exploiting a distinct niche from conical or discoidal species so that multiple forms can co-exist. An example is Dicyema colorum (cap-shaped) which infects Octopus fangsiao in various combinations with D. acuticephalum (conical), D. erythrum (conical), D. misakiense (conical) and D. japonicum (discoidal). Certain rare species, such as Dicyemennea adminicula and Microcyema vespa have highly irregular bodies and calottes. They are proposed to attach to the smooth surfaces of renal appendages, reducing competition between other species present. For example, Conocyema polymorpha (irregular calotte) has been found infecting Octopus vulgaris in the presence of three slightly different conical-calotte species, Dicyema typus, D. paradoxum and Dicyemennea lameere.
The repeated occurrence of particular calotte-type dicyemid species within single host species or individuals indicates convergence on modified head form to allow niche segregation within the restricted renal organ environment. Interestingly, this convergence even extends to the chromidinids, which exhibit similar variation in anterior attachment region morphology. Chromidina elegans has a rounded conical-type ‘head’ region, whereas C. coronata has a ring of elongated cilia around its head, creating a disc-shaped form. Notably, Chromidina elegans and C. coronata have been found together in the Short-finned Squid (Illex coindetii), successfully exploiting distinct regions of the renal organs.
Uncertain origins: What exactly are dicyemids?
 As described above, diycemids and Chromidina provide a stunning example of evolutionary convergence between a multicellular animal and a ciliate protistan, separated by probably a billion years of evolution. However, trying to decide exactly what kind of multicellular beings dicyemids are has been a challenge for biologists since their discovery at the end of the 18th century. Dicyemids were first labelled ‘mesozoans’ as they displayed characteristics intermediate between ciliate protozoans (single celled heterotrophs) and metazoans (multicellular animals). Subsequently, several genes have suggested that they are true metazoans whose bodies have become exceedingly reduced and modified in response to the demands of endoparasitism. Evolutionary or ‘phylogenetic’ trees built using 18SrDNA and b-tubulin indicate that dicyemids are metazoans, and the specific sequence of a Hox gene from Dicyema orientale named DoxC has located them within a large group called the Lophotrochozoa. Lophotrochozoans have either a ciliated mouthpart termed a lophophore for filter feeding (e.g. brachiopods, bryozoans, phoronid worms) or a mobile ‘trochophore’ larval stage bearing ciliate bands (e.g. annelids, molluscs, turbellarian, nemertean and acoel worms). Electron microscopy of the dicyemid Kantharella antarctica (R. Czaker 2000, Anatomical Record vol 259, 52-57) revealed extracellular matrix components (ECM) located inside the cells, indicating either (i) a connection with acoel worms, which lack the typical animal ECM, or (ii) that dicyemids are very primitive animals, even more so than sponges and jellyfish. DoxC sequences suggest that (i) is the more likely scenario, but other features unusual in animals but typical of protistans confuse the issue: dicyemids can engulf food particles in a process called endo- or phago-cytosis, and they also have a double-stranded band of cilia (those of animals are typically triple-stranded). T. Noto and H. Endoh (BioSystems 2004, vol 73, 73-83) propose to resolve the problem by saying that dicyemids are protistans that have acquired lots of molluscan genes by lateral gene transfer, but this idea is tentative and lacks evidence, so for now the assumption that dicyemids are true lophotrochozoans prevails.
As described above, diycemids and Chromidina provide a stunning example of evolutionary convergence between a multicellular animal and a ciliate protistan, separated by probably a billion years of evolution. However, trying to decide exactly what kind of multicellular beings dicyemids are has been a challenge for biologists since their discovery at the end of the 18th century. Dicyemids were first labelled ‘mesozoans’ as they displayed characteristics intermediate between ciliate protozoans (single celled heterotrophs) and metazoans (multicellular animals). Subsequently, several genes have suggested that they are true metazoans whose bodies have become exceedingly reduced and modified in response to the demands of endoparasitism. Evolutionary or ‘phylogenetic’ trees built using 18SrDNA and b-tubulin indicate that dicyemids are metazoans, and the specific sequence of a Hox gene from Dicyema orientale named DoxC has located them within a large group called the Lophotrochozoa. Lophotrochozoans have either a ciliated mouthpart termed a lophophore for filter feeding (e.g. brachiopods, bryozoans, phoronid worms) or a mobile ‘trochophore’ larval stage bearing ciliate bands (e.g. annelids, molluscs, turbellarian, nemertean and acoel worms). Electron microscopy of the dicyemid Kantharella antarctica (R. Czaker 2000, Anatomical Record vol 259, 52-57) revealed extracellular matrix components (ECM) located inside the cells, indicating either (i) a connection with acoel worms, which lack the typical animal ECM, or (ii) that dicyemids are very primitive animals, even more so than sponges and jellyfish. DoxC sequences suggest that (i) is the more likely scenario, but other features unusual in animals but typical of protistans confuse the issue: dicyemids can engulf food particles in a process called endo- or phago-cytosis, and they also have a double-stranded band of cilia (those of animals are typically triple-stranded). T. Noto and H. Endoh (BioSystems 2004, vol 73, 73-83) propose to resolve the problem by saying that dicyemids are protistans that have acquired lots of molluscan genes by lateral gene transfer, but this idea is tentative and lacks evidence, so for now the assumption that dicyemids are true lophotrochozoans prevails.
Cite this web page
Map of Life - "Dicyemids and chromidinids: enigmatic endoparasites"
https://mapoflife.org/topics/topic_432_dicyemids-and-chromidinids-enigmatic-endoparasites/
March 3, 2021

