Topic: Xylem vessels in vascular plants
Vessels are characteristic of the angiosperms, and yet they have evolved independently in several other groups, including the lycophyte Selaginella, horse-tail Equisetum and the enigmatic Gnetales.
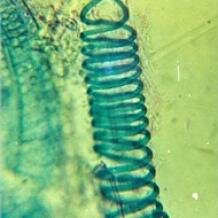 Vascular plants or ‘tracheophytes’ have specialised tissue, termed xylem and phloem, for conducting water (plus solutes) and organic nutrients respectively. Xylem cells are elongated and connected end to end to form a tubular water-transport system throughout the plant, continuously replacing the large amount of water lost by evaporation, water that is essential for both photosynthesis (CO2 + H2O → sugars) and to maintain turgor pressure. The main kinds of xylem are tracheids and vessel elements, the latter being more specialised for efficient water conduction, reducing the costs of water loss by evapo-transpiration.
Vascular plants or ‘tracheophytes’ have specialised tissue, termed xylem and phloem, for conducting water (plus solutes) and organic nutrients respectively. Xylem cells are elongated and connected end to end to form a tubular water-transport system throughout the plant, continuously replacing the large amount of water lost by evaporation, water that is essential for both photosynthesis (CO2 + H2O → sugars) and to maintain turgor pressure. The main kinds of xylem are tracheids and vessel elements, the latter being more specialised for efficient water conduction, reducing the costs of water loss by evapo-transpiration.  Vessels are characteristic of the angiosperms, the most advanced and diverse group of plants, and yet they have evolved independently in several other groups. For example, many species of the lycophyte Selaginella have vessels, and lycophytes branched from euphyllophytes (the lineage leading to angiosperms) in the early Devonian, over 400 million years ago. A number of primitive euphyllophytes have vessels, including the monilophyte Equisetum (horse-tail) and two seed-ferns, Marsilea and Pteridium. Among the gymnosperms, xylem vessels have been discovered in members of the Gnetales (Gnetum, Ephedra, Welwitschia), as well as in Permian fossils of Gnetum-like ‘gigantopterids’ (Gigantopteris, Gigantoclea). The following paragraphs explain more about what tracheids and vessel elements are and how vessels have evolved by convergent evolution.
Vessels are characteristic of the angiosperms, the most advanced and diverse group of plants, and yet they have evolved independently in several other groups. For example, many species of the lycophyte Selaginella have vessels, and lycophytes branched from euphyllophytes (the lineage leading to angiosperms) in the early Devonian, over 400 million years ago. A number of primitive euphyllophytes have vessels, including the monilophyte Equisetum (horse-tail) and two seed-ferns, Marsilea and Pteridium. Among the gymnosperms, xylem vessels have been discovered in members of the Gnetales (Gnetum, Ephedra, Welwitschia), as well as in Permian fossils of Gnetum-like ‘gigantopterids’ (Gigantopteris, Gigantoclea). The following paragraphs explain more about what tracheids and vessel elements are and how vessels have evolved by convergent evolution.
WHAT ARE XYLEM TRACHEIDS AND VESSEL ELEMENTS?
 Tracheids most likely evolved from ‘hydroid’ cells of bryid mosses, the most primitive of land plants. Hydroids are elongated cells that conduct water and solutes, being strengthened with polyphenols and at maturity emptied of cell contents. Within the bryophytes (liverworts, hornworts and mosses) it appears that water conducting cells are themselves convergent, as other forms of water-conduction cell have been noted in the moss Takakia and several liverworts (e.g. Haplomitrium, Moerckia and Pallavicinia). Distinct, hydroid-like conducting cells occur in extinct Silurian ‘pro-tracheophytes’ such as Aglaophyton and Horneophyton, strengthened by lignin-like polyphenols in the cell wall.
Tracheids most likely evolved from ‘hydroid’ cells of bryid mosses, the most primitive of land plants. Hydroids are elongated cells that conduct water and solutes, being strengthened with polyphenols and at maturity emptied of cell contents. Within the bryophytes (liverworts, hornworts and mosses) it appears that water conducting cells are themselves convergent, as other forms of water-conduction cell have been noted in the moss Takakia and several liverworts (e.g. Haplomitrium, Moerckia and Pallavicinia). Distinct, hydroid-like conducting cells occur in extinct Silurian ‘pro-tracheophytes’ such as Aglaophyton and Horneophyton, strengthened by lignin-like polyphenols in the cell wall.  True tracheids first appear in Devonian fossil taxa such as Rhynia, and are found throughout the lycophytes (plants with ‘lycophylls’ rather than true leaves) and euphyllophytes (plants with typical, ‘true’ leaves). Tracheids are elongated cells up to 80µm wide with secondary, lignified cell walls. In early-formed ‘proto-xylem’, secondary lignin is deposited in rings or spirals around the primary cell wall, strengthening while permitting expansion of un-lignified regions during cell growth. The primary cell wall is made of cellulose but is interrupted by 50nm wide inter-cellular connections called plasmodesmata, through which small molecules may pass. In later-formed ‘meta-xylem’ or ‘secondary xylem’ (in woody plants) secondary lignin covers the whole primary cell wall, except at gaps called ‘pit-pairs’, where small regions of un-lignified primary wall from two adjacent cells are opposed at the middle lamella. Pit-pairs are concentrated at axial junctions (end walls) between cells, forming a pitted region called a porous pit membrane or plate. When mature, tracheids are subject to loss of protoplast (nucleus and cytoplasm) and hence cell death, creating an open structure for water flow, retarded only by the thin cellulose barrier of the porous pits. Functional tracheal conduits are surrounded by support and storage cells, including parenchyma, fibers and sclereids.
True tracheids first appear in Devonian fossil taxa such as Rhynia, and are found throughout the lycophytes (plants with ‘lycophylls’ rather than true leaves) and euphyllophytes (plants with typical, ‘true’ leaves). Tracheids are elongated cells up to 80µm wide with secondary, lignified cell walls. In early-formed ‘proto-xylem’, secondary lignin is deposited in rings or spirals around the primary cell wall, strengthening while permitting expansion of un-lignified regions during cell growth. The primary cell wall is made of cellulose but is interrupted by 50nm wide inter-cellular connections called plasmodesmata, through which small molecules may pass. In later-formed ‘meta-xylem’ or ‘secondary xylem’ (in woody plants) secondary lignin covers the whole primary cell wall, except at gaps called ‘pit-pairs’, where small regions of un-lignified primary wall from two adjacent cells are opposed at the middle lamella. Pit-pairs are concentrated at axial junctions (end walls) between cells, forming a pitted region called a porous pit membrane or plate. When mature, tracheids are subject to loss of protoplast (nucleus and cytoplasm) and hence cell death, creating an open structure for water flow, retarded only by the thin cellulose barrier of the porous pits. Functional tracheal conduits are surrounded by support and storage cells, including parenchyma, fibers and sclereids.  It has been suggested that tracheids evolved as Carboniferous and Permian plants were colonising the land and atmospheric CO2 levels were decreasing. This resulted in selection for efficient water transport (and better stomatal regulation) to counteract elevated water loss by evapo-transpiration from photosynthesising leaves.
It has been suggested that tracheids evolved as Carboniferous and Permian plants were colonising the land and atmospheric CO2 levels were decreasing. This resulted in selection for efficient water transport (and better stomatal regulation) to counteract elevated water loss by evapo-transpiration from photosynthesising leaves.
Vessel elements are more specialised and provide even more efficient water conduction than tracheids due to having a completely open structure. They have a diameter up to 500µm and by virtue of being multi-cellular, conduits can reach lengths in excess of 10m. Vessels have evidently developed by modification of the ancestral genetic programs employed for tracheid formation. Like tracheids, they are elongated, secondarily lignified cells that lack living contents at maturity. At the end walls of vessels, cell walls of pits completely break down when the protoplast is lost, leaving an open ‘perforation plate’ through which water can pass freely. The perforation plate is termed ‘foraminate’ if the open pores are rounded, ‘scalariform’ if pores are elongated and aligned like a ladder, ‘reticulate’ if they are arranged in a net-like pattern, and ‘simple’ if the whole plate is open. Initial selection for these high hydraulic conductivity structures may have been associated with a further decrease in atmospheric CO2 levels in the Cretaceous, favouring better water conduction to manage elevated transpiration in the groups (e.g. angiosperms and gnetophytes) that were diversifying at that time.
CONVERGENT EVOLUTION OF XYLEM VESSELS
 Xylem vessels with perforation plates of various forms have evolved at least seven times in the plants: once in the lycophytes and six times in the euphyllophytes. Lycophytes (with ‘lycophylls’) and euphyllophytes (with true leaves) diverged from one another approximately 420 Ma, in the Silurian.
Xylem vessels with perforation plates of various forms have evolved at least seven times in the plants: once in the lycophytes and six times in the euphyllophytes. Lycophytes (with ‘lycophylls’) and euphyllophytes (with true leaves) diverged from one another approximately 420 Ma, in the Silurian.
The lycophyte group Selaginellales is known from the Devonian, about 390 Ma, and includes the extant genus Selaginella, where species with similarly shaped or ‘homophyllous’ leaves have meta-xylem vessel elements. The perforation plates of Selaginella are generally scalariform (e.g. S. oregana, S. rupestris, S. arizonica), but S. bigelonii shows simple plates, where the whole end wall is lost. S. spinosa is unique among homophyllous selaginellids in having only tracheids, as is the case for all remaining ‘heterophyllous’ members of Selaginellales (e.g. S. chrysorhizos, S. grandis).
 A diverse group of euphyllophytes termed the ‘monilophytes’ evolved in the early-mid Devonian, including various seed-less ferns and the Equisetales, or horse-tails. Species of Equisetum have been found to contain two types of xylem vessel, one formed in a unique way by rupture of cell walls as outgrowths invade from adjacent cells, and another formed by typical modification of tracheids in subterranean stems. Equisetum tracheids have tapering end walls, and around 20-50% porous pit membranes are completely broken down, leaving a reticulate or simple vessel plate.
A diverse group of euphyllophytes termed the ‘monilophytes’ evolved in the early-mid Devonian, including various seed-less ferns and the Equisetales, or horse-tails. Species of Equisetum have been found to contain two types of xylem vessel, one formed in a unique way by rupture of cell walls as outgrowths invade from adjacent cells, and another formed by typical modification of tracheids in subterranean stems. Equisetum tracheids have tapering end walls, and around 20-50% porous pit membranes are completely broken down, leaving a reticulate or simple vessel plate.
 Seed ferns or ‘pteridosperms’ diversified in the Carboniferous and include at least two independent lineages with xylem vessels: the terrestrial fern Pteridium (bracken) and the aquatic fern Marsilea. Pteridium xylem vessels occur throughout the plant and have scalariform perforation plates. Three species of Marsilea (M. quadrifolia, M. drummondii, M. hursuta) have vessels that are on average 3.6mm long and 30µm wide, occur only in the roots, and have perforation plates that vary in form from scalariform to simple.
Seed ferns or ‘pteridosperms’ diversified in the Carboniferous and include at least two independent lineages with xylem vessels: the terrestrial fern Pteridium (bracken) and the aquatic fern Marsilea. Pteridium xylem vessels occur throughout the plant and have scalariform perforation plates. Three species of Marsilea (M. quadrifolia, M. drummondii, M. hursuta) have vessels that are on average 3.6mm long and 30µm wide, occur only in the roots, and have perforation plates that vary in form from scalariform to simple.
 Gymnosperms originated in the mid-Carboniferous (around 325 Ma), and include two groups with vessel elements: the Gnetales and Gigantopteridales, the latter being of somewhat uncertain affinity. Living members of the Gnetales include Gnetum, Ephedra and Welwitschia, all of which have meta-xylem and secondary xylem vessels with foraminate perforation plates (i.e. composed of many rounded pits). Our knowledge of gigantopterids comes from Permian fossils from China and the USA (e.g. Gigantopteris, Gigantoclea), and evidence indicates a liana-type plant with tendril-like appendages on the stem and large leaves for capturing light within the forest canopy. Gigantopterid xylem vessels have foraminate perforation plates like the Gnetales, and are very wide (150-300µm average diameter) and long (5-7mm average length). The large size and inner stem localisation of these xylem vessels parallels those of angiosperm lianas, and may represent a shared adaptation for increased conduction efficiency within these canopy-dwelling plants.
Gymnosperms originated in the mid-Carboniferous (around 325 Ma), and include two groups with vessel elements: the Gnetales and Gigantopteridales, the latter being of somewhat uncertain affinity. Living members of the Gnetales include Gnetum, Ephedra and Welwitschia, all of which have meta-xylem and secondary xylem vessels with foraminate perforation plates (i.e. composed of many rounded pits). Our knowledge of gigantopterids comes from Permian fossils from China and the USA (e.g. Gigantopteris, Gigantoclea), and evidence indicates a liana-type plant with tendril-like appendages on the stem and large leaves for capturing light within the forest canopy. Gigantopterid xylem vessels have foraminate perforation plates like the Gnetales, and are very wide (150-300µm average diameter) and long (5-7mm average length). The large size and inner stem localisation of these xylem vessels parallels those of angiosperm lianas, and may represent a shared adaptation for increased conduction efficiency within these canopy-dwelling plants.
 The angiosperms or flowering plants diversified throughout the Cretaceous to become the most speciose and diverse group alive today. Evidence suggests that the New Caledonian shrub Amborella represents a particularly primitive angiosperm, and it does not have xylem vessels. All other angiosperms, however, do posses xylem vessels, and it has been suggested that this key feature contributed significantly to their radiation as CO2 levels declined during the Cretaceous.
The angiosperms or flowering plants diversified throughout the Cretaceous to become the most speciose and diverse group alive today. Evidence suggests that the New Caledonian shrub Amborella represents a particularly primitive angiosperm, and it does not have xylem vessels. All other angiosperms, however, do posses xylem vessels, and it has been suggested that this key feature contributed significantly to their radiation as CO2 levels declined during the Cretaceous.
Cite this web page
Map of Life - "Xylem vessels in vascular plants"
https://mapoflife.org/topics/topic_450_xylem-vessels-in-vascular-plants/
November 25, 2020

